|
Development of Plastics Injection Molded Medical Device: A
Systematic Approach is Key to Success
Production Tooling For Mass
Manufacturing :
Based on the learning of proto
tooling, final product drawings are released for
development of production tooling.
Mold and Process validation is
validated scientifically to ensure consistent device
quality and performance.
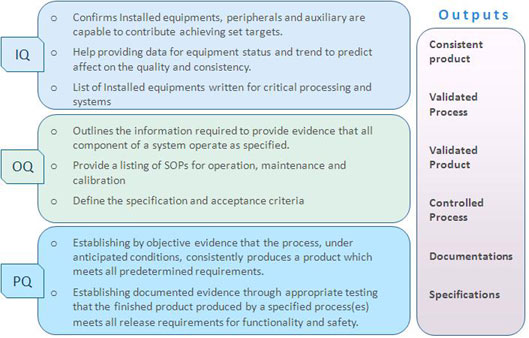
Validation protocols for
Installation Qualifications, Operational qualifications
and Performance qualifications should be designed and
performed to have consistent quality device at optimum
efficiency throughout product life cycle.
Data Transfer & Mass
Manufacturing :
Implementing a quality system
is a big and critical task for new medical devices. A
quality system starts by defining, documenting, and
formally approving and releasing document systems, both
products specific and administrative. This is a resource
demanding and time consuming task and It is done in
parallel with product development.
Data transfer is very critical
stage in the device development process. Device Master
Record (DMR), Design History File (DHF), Manufacturing
drawings, Final product testing procedures and other SOP
has been released to manufacturing team.
Regulatory submissions are
also been done parallel with device development process.
After having all necessary
regulatory and statutory approvals, the last phase of
device development is the mass manufacturing and product
launch in market.
How can we contribute to
plastics medical device development?
Credence Management
corporation is a Plastics Technical Project Management and
execution firm, backed by qualified and experienced
professionals, that offers a complete range of customized
technical support for development of plastics medical
devices and healthcare products.

Page
1 :
2 :
3 :
4 :
5 |
