|
Supplier Quality Management For Medical Device
Manufacturers: A Critical Step Towards Global Regulatory
Compliance
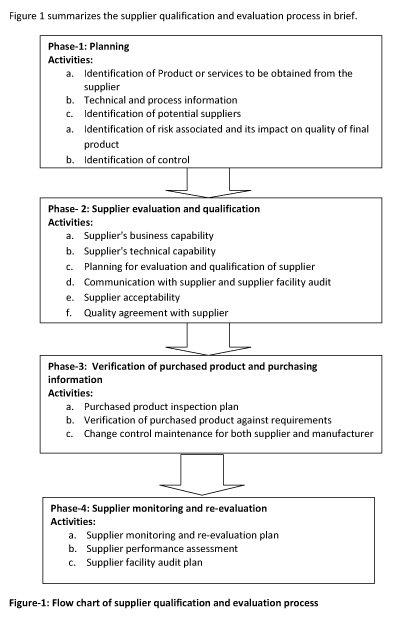
3. Verification Of Purchased Product And Purchasing
Information
Supplier performance assessment is a continuous process.
It is done by verifying the purchased product. Establish
inspection plan for each batch or lot of supplied product
to verify it against the product requirements. The extent
of these verification activities is based on the supplier
evaluation results and proportionate to the risks
associated with the purchased product.
When any changes occur to the purchased product, the
manufacturer should determine whether these changes affect
the final product quality or not. Certificate of analysis
must be included into the purchasing information for each
supplied batch or lot. It mentions the product
specification, acceptance criteria, procedures or
equipment used, whenever necessary. This purchasing
information shall be maintained for the purpose of
traceability. Any changes occurred in the processing or
specification of product that may affect the final product
quality, supplier should notify to manufacturer in this
regard by written communication. Change control should be
maintained by both supplier and manufacturer.
4. Supplier Monitoring And Re-Evaluation
After selecting supplier for any particular product or
services, Supplier monitoring and re-evaluation is a
continuous process. It is required to be done in a timely
manner. Make a qualification plan for monitoring and
re-evaluation of suppliers. It should be separate for each
supplier. Assess the supplier performance during this
period and give the ranking to it. Performance is checked
based on supplier’s ability to deliver good products,
delivery time, supplier initiated issues, supplier-
initiated change requests, supplier facility audit, change
control etc. Monitor the performance of supplier for
meeting the requirements of the purchased product. These
results of the monitoring provide an input into the
supplier re-evaluation process. Non-fulfillment of
purchasing requirements shall be addressed with the
supplier; discuss the risk associated with the purchased
product and its failure to comply with applicable
regulatory requirements.
• Supplier Facility Audit:
Supplier audit constitute major part of the supplier
qualification programme. It should also be conducted on
regular bases as continuous evaluation of supplier. Make
audit strategy and prioritize the audit based on risk. One
manufacturer may have number of suppliers. Each supplier
facility cannot be audited. Hence, decide it based upon
quantifying supplier risk, accounting for both performance
and criticality; we can effectively prioritize issues that
require the most attention. It is advisable to treat these
external risks similar to internal insufficiencies or
gaps. Employing CAPA or deviation management techniques,
as you would in-house, will mitigate supplier risk while
also avoiding the same issues from arising in the future.
Supplier audit frequency is also to be decided in audit
plan.
These all requirement fulfills the supplier quality
management requirement and allows medical device
manufacturer for global regulatory compliance. Global
regulatory compliance is important because in today’s
world of globalized supply and demand networks, companies
need to efficiently optimize the supply base given a broad
set of requirements that go well beyond cost. To
effectively do this, companies should begin to use a risk
based approach that looks at both the criticality of a
supplier and the likelihood of failure of a supplier. By
applying standardized risk and audit tools, the long term
successes of initiatives around supplier quality are much
more likely to succeed.
Page
1 :
2 :
3
|
