|
Bio-Compatible, Lightweight Polycarbonate Utilized in
Respiratory Device
Medical technology continues
to advance by leaps and bounds but bringing life-saving
products to market requires more than medical
breakthroughs: it also requires materials that can meet
the rigorous challenges these applications demand.
Polycarbonate resins and blends developed by Covestro help
bring medical and healthcare applications to life.
Imagine not being able to
climb stairs or stand and wash dishes without getting
winded. Unfortunately this is a common reality for
individuals suffering from respiratory conditions.
Ventilators can help reduce the work of breathing by
unloading the ancillary respiratory muscles, but are often
bulky and heavy, creating additional user limitations.
A better solution by reducing
the overall size of the ventilation system to support
patient mobility and independence will be the desire from
chronic respiratory disease patients.
US respiratory OEM Breathe
Technologies Inc. selected medical Makrolon® from Covestro
to achieve the R&D goals for their Non-Invasive Open
Ventilation System (NIOV).
The main properties of
Makrolon® 2858 polycarbonate are :
• Medium viscosity
• Medical device grade
• Suitable for ETO and steam sterilization at 121 °C
• Easy release
• Available in transparent and opaque colors
• Biocompatible according to many ISO 10993-1 test
requirements
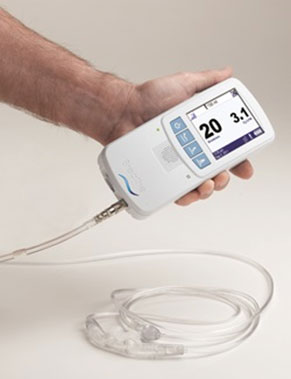 |
Makrolon® polycarbonate from
Covestro helps Breathe Technologies Inc. achieve the
high-performing yet portable device design. The Breathe
NIOV System is a one-pound, non-invasive mechanical
ventilator that can be used in home and institutional
settings. Breathe Technologies explains that the device
utilizes novel venturi principle technology in a
comfortable facial interface that can be worn while
talking and exercising.
The patient interface and
air/oxygen path components utilize injection-molded
Makrolon® 2858. This grade is suitable for ETO and steam
sterilization at 121 Deg C and is biocompatible according
to many ISO 10993-1 test requirements, making it an ideal
choice for medical devices. Makrolon® 2858 also features
easy release and medium viscosity and is available in
transparent and opaque colors. The device components made
of Makrolon® are intricate in design and require optimal
functionality for patient safety.
The Breathe NIOV System has
received five 510(K) clearances from the U.S. FDA for use
with compressed oxygen or compressed air for home and
institutional use, and includes invasive and noninvasive
circuits.
The needs are real, and the
ideas to meet those needs are here…
Covestro helps them
materialize.
Local Contact : Covestro India:
pcsproductservices@covestro.com
www.covestro.in
|
About Covestro :
With 2017 sales of EUR 14.1 billion, Covestro is among the
world’s largest polymer companies. Business activities are
focused on the manufacture of high-tech polymer materials
and the development of innovative solutions for products
used in many areas of daily life. The main segments served
are the automotiveconstruction, wood processing and
furniture, and electrical and electronics industries.
Other sectors include sports and leisure, cosmetics,
health and the chemical industry itself. Covestro has 30
production sites worldwide and employs approximately
16,200 people (calculated as full-time equivalents) at the
end of 2017..
|
