|
Low Risk, Patient
Friendly Microneedle Arrays: An Emerging
Medical Device for Enhanced Local/Systemic, Transdermal
Drug Delivery
Transdermal drug
delivery by the breakage of stratum corneum
MNs were conceptualized
in the early 1970’s as a simple idea to enhance the skin
permeability of hydrophilic and large size molecules
therapeutics by breaking the skin barrier layer
physically. Since the first generation of MNs was
fabricated out of silicon using micro-electro mechanical
systems (MEMS) in 1998 shown in Figure 2 , various types
of MNs have been designed and evolved with improved
features; such as solid metal MNs, hollow metal MNs,
hollow glass MNs, solid biodegradable polymer, and so
forth. These MNs enabled various types of transdermal drug
delivery. Transdermal drug delivery with MNs has been
extensively investigated with various drugs, most of which
have hydrophilic properties that are not applicable to
passive diffusion based transdermal patch systems.
Alza Corp. designed the
drug coated micro-projection array system, Macroflux® as
shown in Figure 3. They fabricated a titanium
micro-projection array which is inserted with coated drug
into skin. Zosano Pharma™, Inc. developed a parathyroid
hormone (PTH) transdermal delivery system with Macroflux®
technology for osteoporosis and Phase II clinical trials
have been completed.

Figure 3
Transdermal drug delivery with Macroflux® technology using
drug coated MNs
For hollow microneedle
applications, Nanopass Technologies LTD developed
MicronJet, an intradermal system for proteins and vaccines
requiring minimal expertise for administration. The device
consists of MicroPyramids made of pure silicon crystals
which are mounted on a standard syringe for the
replacement of a conventional hypodermic needle.
Currently, two pilot clinical studies have been completed
to assess the safety and efficacy of MicronJet as shown in
Figure 4.
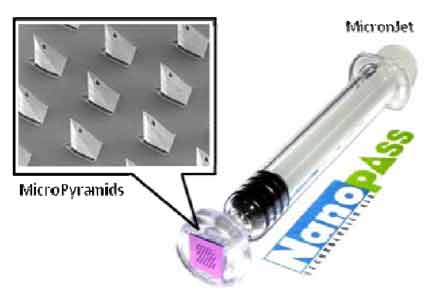
Figure 4
MicronJet intradermal self-administration system
Types of microneedle
1. Solid microneedle
2. Coated MNs
3. Hollow microneedle
4. Dissolvable microneedle
Solid microneedle
MNs can be used as a
pretreatment for pore formation in the skin (Figure 5,
skin pretreatment). Sharp MNs penetrate into or scrape the
skin in order to make holes through which drugs can
transport, either for local effect in the skin or for
systemic delivery after uptake by skin capillaries. The
drug can be applied to the
skin surface over the pores using a drug-loaded patch, as
is commonly used in conventional transdermal drug
delivery, or using a semisolid topical formulation, such
as an ointment, cream, gel or lotion, as is commonly used
for other skin treatments.
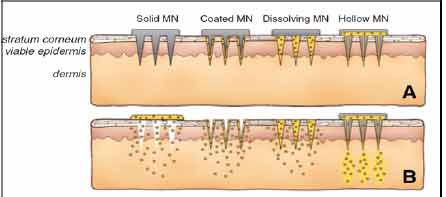
Figure 5 Methods of
drug delivery to the skin using MNs (MN). MNs are first
applied to the skin (A) and then used for drug delivery
(B). Solid MNs are used as a pretreatment, after which
drug can diffuse through residual holes in skin from a
topical formulation (solid MN). After insertion of
drug-coated MNs into the skin, the drug coating dissolves
off the MNs in the aqueous environment of the skin (coated
MN). Drug-loaded MNs are made of water-soluble or
biodegradable materials encapsulating drug that is
released in the skin upon microneedle dissolution
(dissolving MN). Hollow MNs are used to inject liquid
formulations into the skin (hollow MN).
Coated MNs
Solid MNs can be used not only as
piercing structures, but also as vehicles to carry and
deposit drug within the skin or other tissue (Figure 5,
drug-coated MNs). This can be done by coating MNs with a
drug in a formulation suitable for coating and subsequent
dissolution. In this way, the desired dose of the drug is
delivered into tissue quickly upon insertion of the MNs.
The drug dose that can be administered this way is limited
to the amount that can be coated onto the tip and shaft of
the MNs, which is typically less than 1 mg for small
microneedle arrays.
Hollow microneedle
Hollow MNs provide a defined conduit
for drug delivery into the skin or other tissue. Similar
to hypodermic injection, hollow MNs enable pressure-driven
flow of a liquid formulation (Figure 5, hollow MNs).
Pressure, and thereby flow rate, can be
modulated for a rapid bolus injection, a slow infusion or
a time varying delivery rate. The liquid formulation may
simplify use of existing injectable formulations for
delivery using MNs, but misses the opportunity of solid
microneedle delivery methods to administer dry-state drug
formulations without reconstitution to improve drug
stability and the patient convenience of a patch-based
delivery method.
|
