|
Quality Requirements For
Medical Textiles, PPES / Face Masks
|

K. R. Navaneethakrishnan, MSc, ERT
Assistant Director,
GLR Laboratories Private Limited, Chennai |
Personal Protective Equipment (PPE)
have become a mandatory part of our life, given the
unforgiving grudge of the global pandemic on the human
race. PPEs play a significant role in preventing
transmission of infectious agents, not only in hospitals,
but also in various activities such as cleaning, waste
management and safe burials, and community care related to
the outbreak. March 2020, saw an incredible increase in
the import of personal protective equipment kits by
various countries which led to a global shortage of
supplies. In India, the PPE industry grew a stunning 56
times in the last couple of months. As many as 600
companies in India are registered to manufacture the
protective gears. |
As per a notification released by MoH &FW
vide S.O. 648(E), PPE kits are considered as a medical device,
in pursuance of sub-clause (iv) of clause (b) of section 3 of
the Drugs and Cosmetics Act, 1940 (23 of 1940). CDSCO
notification dated 03 Sep 2020 listed out 24 devices as
Personal Protective Equipment’s.
Some examples of PPE include :
-
Face shield,
-
Face Mask (Disposable, Surgical, N95),
-
Gowns (surgical gowns, isolation gowns,
surgical isolation gowns, nonsurgical gowns, procedural gowns,
and operating room gowns)
-
Gloves (Latex surgical gloves, Non-latex
surgical gloves, Nitrile gloves, Examination gloves)
With the increase in the demand for PPEs, it is necessary that
manufacturers lay adequate emphasis on the selection of raw
materials as the quality of finished device is heavily
dependent on these raw materials.
Although PPEs are used for personal protection, they
themselves may cause adverse reactions. Adverse reactions that
are commonly reported are acne, skin rash and contact
dermatitis which may arise from some components (adhesives,
rubber straps or metal clips) of the N95 mask. Skin reactions
to gloves included complaints of dry skin, itch, and rash.
Type I immunoglobulin Emediated natural rubber latex
hypersensitivity is an important, often undiagnosed,
occupational health hazard for healthcare workers, especially
in those with high exposure.
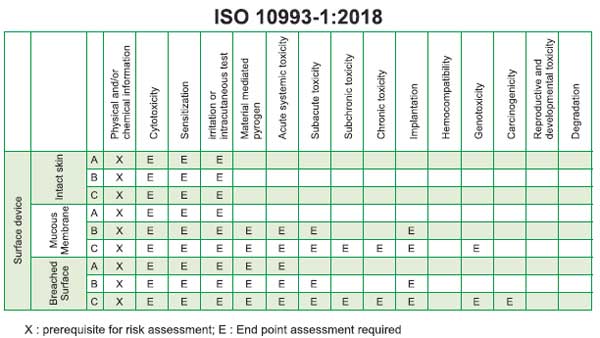
Biocompatibility tests (ISO 10993) for
PPE / Medical Textiles
One of the quality parameters that certify
a medical device as safe is its property of being
biocompatible.
Biocompatibility tests are recommended for
medical devices that come into direct contact or indirect
contact with the human body in order to determine the
potential for an unacceptable adverse biological response
resulting from contact of the component materials of the
device with the body. US FDA Guidance document issued on 04
September 2020 states that the term “human body” refers to
either patient tissues or the clinical practitioner. Therefore
"masks or gloves intended for protective purposes by clinical
practitioners should be assessed for biocompatibility."
Testing for biocompatibility considerations
should be performed on the device in its final and finished
form.
As per ISO 10993-1:2018, majority of PPE
devices are classified as Surface Contacting Devices with
contact duration lesser than 24 hours. Below is a snapshot of
the testing requirements for surface (Skin, Mucous membrane,
Breached/compromised surfaces) contacting medical devices
based on their contact surface and the following
biocompatibility tests are usually recommended for PPEs. The
principle behind each of these tests and the results they
produce is briefly discussed below:
• Cytotoxicity test (ISO 10993-5:2009)
• Irritation/ Intracutaneous reactivity test (ISO
10993-10:2010)
• Sensitization test (ISO 10993-10:2010)
|
|
|
Advertisers' Index
|
|
Accuprec Research Labs Pvt. Ltd., India |
|
Ambica Medicare Engineering, India |
|
Nu-Vu Conair Pvt. Ltd., India |
|
Divya Steri Solutions Pvt. Ltd., India |
|
ET Elastomer Technik, Germany |
|
Eewa Engineering Co. Pvt. Ltd., India |
|
Ineos Styrolution India Ltd., India |
|
I-Kare Polyalloys Pvt. Ltd., India |
|
KLJ
Group, India |
|
Lubrizol Advanced Materials India Pvt.
Ltd. |
|
Kuraray India Pvt. Ltd., India |
|
Maider Medical Industry Equipment Ltd.China |
|
Medicall 2019, India |
|
Ferromatik Milacron India Pvt. Ltd., India |
|
GLR Laboratories
Pvt. Ltd., India |
|
Pashiba Lifescience, India |
|
Plastivision India |
|
Pradeep Surgipack, India |
|
PVC Colouring Compounding & Processing,
India |
|
Qosina,
USA |
|
Raumedic AG |
|
SMC Medical Manufacturing Pvt. Ltd.,
India |
|
Sterimed Medical Devices (P) Ltd., India |
|
Steri Techno Fab, India |
|
Tekni-Plex India Pvt. Ltd., India |
|
Twist Engineering Works,India |
|
Yuhuan Shengjiu Mould Co., Ltd.,
China |
|
Airways Surgical Pvt. Ltd., India |
|
Alpha Medicare and Devices Ltd., India |
|
Alpha Therapeutics Pvt. Ltd., India |
|
Ami
Polymer Pvt. Ltd., India |
|
Angiplast Pvt. Ltd., India |
|
Appasamy Associates, India |
|
Beacon Plastics, India |
|
Delux Surgical, Inida |
|
Ignisol Mediplas Corporation, India |
|
Jain Rubbers Pvt. Ltd., India |
|
Operon Strategist, India |
|
R.R. Patel Gases (P) Ltd., India |
|
Proven Trade Contacts, India
|
|
Sanidhya
Enterprise, India |
|
Surgi Pack India Pvt. Ltd. |
|
Unikal Consultants, India |
|
Vinit Performance Polymers Pvt. Ltd., India |
|
Aircity, India |
|
Amigo Surgi Care
Pvt. Ltd., India |
|
Angel Products, India |
|
Apex Medical Devices, India |
|
Jimit Medico Surgicals Pvt. Ltd. |
|
Kavya Packaging, India |
|
Life-O-Line Technologist, India |
|
Mesco Surgical, India |
|
Morrisons
Lifecare Pvt. Ltd., India |
|
National Healthcare, India |
|
Pharmadocx, Inida |
|
S. Nath & Co., India |
|
Unikal Consultants, India |
|
Venus
Industries,India, Mobile : 9825747495 |
|
Zinkal Products, India |
|
