|
Go to Market Strategies
for Medical Device Start-ups and Entrepreneurs in India

Bhupesh Sood
CEO, SEC Global Consulting |

Tapan Kumar Patel
Principal Consultant, SEC Global Consulting |
Overview
India is the 4th largest market for medical devices in
Asia. Medical device sector is projected to register a
CAGR of 14.8% and is expected to reach $11.86 bn in
2021-22.
As stated by the new market research report on medical
device outsourcing, the United States represents the
largest market worldwide. Asia-Pacific is forecast to
record the fastest CAGR of 14.9% over the analysis
period- 2020. Less expensive production of devices
coupled with adherence to stringent international
quality standards marks the emergence of Asia as the
most desirable production hub. Asian countries,
specifically India and China, are emerging as attractive
low-cost destinations for leading medical devices OEMs.
India’s medical devices industry is poised for
significant growth in the next five years: The market
size is expected to reach $50 bn by 2025. 100% FDI is
allowed under the automatic route for both brownfield
and greenfield setups. Strong FDI inflows reflect the
confidence of global players in the Indian market.
Since April
2000, $2.1 bn in FDI. Of this, $600 mn was
received in the last 5 years. Singapore, United States,
Europe, and Japan are key investors.
Equipment & Instruments, Consumables and Implants have
attracted most FDI . The Government of India has taken
several steps to ensure the growth of a vibrant
ecosystem of medical devices manufacturing in India over
the past 5 years:
-
Recognized Medical Devices as a sunrise sector under
Make in India campaign, 2014
-
The Medical Devices Rule of 2017
-
Adopted risk-based classification based on GHT
guidelines: Classes A, B, C, D
-
Perpetual licences for manufacturers
-
The Medical Devices Amendment Rules of 2020 bring all
medical devices in India under regulation as drugs. The
medical devices industry in India consists of large
multinationals as well as small and medium
enterprises (SMEs) growing at an unprecedented scale.
-
The current market size of the medical devices industry
in India is estimated to be $11 bn.
Around 65% of the manufacturers in India are mostly
domestic players operating in the consumables segment
and catering to local consumption with limited exports.
Large Multinational Corporations lead the high
technology end of the Medical Devices market with
extensive service networks. There are 750–800 domestic
Medical Devices manufacturers in India. The
manufacturing is developing in its scale and geography:
there are six Medical devices manufacturing “clusters”
in the country. Clusters have “Medical Device Parks”
developing around them: states have committed to set-up
dedicated industrial parks where efficient domestic
manufacturing at lower costs. In 2019, Andhra Pradesh,
Telangana, Tamil Nadu, and Kerala have got in
principal approval from Government of India for
new medical devices parks.
The Process from Ideation to Customer (i.e. Patient)
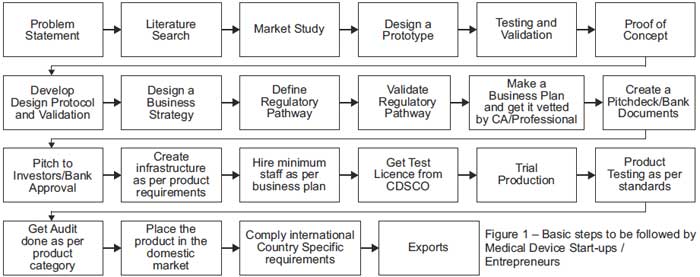 |
Understanding the applicable standards
When any Medical Device Start-ups /
Entrepreneurs is considering to venture into the medical
device manufacturing he has to consider many factors
before the product is placed in the market. He has to
work a lot on the compliance requirements that are
applicable to the product, that is proposed to be placed
on the Indian market. Many such manufacturers will fail
if they are not keeping an eye on the product related
compliance requirements.
Example 1 – Infusion Set – If you
plan to make an infusion set, say Sterile Single Use
Scalp Vein (Winged Needle) Infusion Set, then BIS
requirements that need to be followed are IS 16097:2013.
In addition to these, you also need to understand the
sterilisation requirements along with testing and
laboratory requirements.
Example 2 – Blood Transfusion Set
- Similarly, say Blood Transfusion Set (for Single Use
Only), the requirements shall be ISO 8536-4 compliant
spike, ISO 594/1 & ISO 594/2 compliant end connection.
In addition to these, you also need to understand the
sterilisation requirements. along with testing and
laboratory requirements. |
|
|
Advertisers'
Index
|
|
Accuprec Research Labs Pvt. Ltd., India |
|
Ambica Medicare Engineering, India |
|
Nu-Vu Conair Pvt. Ltd., India |
|
Celanese Corporation, India |
|
CLS Pvt.
Ltd., India |
|
Carclo Technical Plastics Pvt. Ltd.,
India |
|
Covstro India |
|
ET Elastomer Technik, Germany |
|
Eewa Engineering Co. Pvt. Ltd., India |
|
Ineos Styrolution India Ltd., India |
|
I-Kare Polyalloys Pvt. Ltd., India |
|
KLJ
Group, India |
|
Lubrizol Advanced Materials India Pvt.
Ltd. |
|
Lyondellbasell, India |
|
Mediscient Devices (OPC) Pvt. Ltd., India |
|
Milliken & Company, India |
|
Maider Medical Industry Equipment Ltd.China |
|
Mega Compound Co. Ltd., China |
|
Milacron India Pvt. Ltd., India |
|
GLR Laboratories
Pvt. Ltd., India |
|
HighRichja Precision Extrusion Machinery Co. Ltd., China |
|
PVC Colouring Compounding & Processing,
India |
|
Qosina,
USA |
|
Raumedic AG |
|
San
Printech Pvt. Ltd., India |
|
Schottli, Switzerland |
|
SMC Medical Manufacturing Pvt. Ltd.,
India |
|
Steri Techno Fab, India |
|
Tekni-Plex India Pvt. Ltd., India |
|
Twist Engineering Works,India |
|
Airways Surgical Pvt. Ltd., India |
|
Alpha Medicare and Devices Ltd., India |
|
Alpha Therapeutics Pvt. Ltd., India |
|
Angiplast Pvt. Ltd., India |
|
Beacon Plastics, India |
|
Delux Surgical, Inida |
|
Jain Rubbers Pvt. Ltd., India |
|
New
Vimko Plastics, India |
|
Operon Strategist, India |
|
R.R. Patel Gases (P) Ltd., India |
|
SEC Global Consulting & Initiative LLP,
India |
|
Surgi Pack India Pvt. Ltd. |
|
Vinit Performance Polymers Pvt. Ltd., India |
|
Amigo Surgi Care
Pvt. Ltd., India |
|
Apex Medical Devices, India |
|
Jimit Medico Surgicals Pvt. Ltd. |
|
Life-O-Line Technologist, India |
|
Mesco Surgical, India |
|
Morrisons
Lifecare Pvt. Ltd., India |
|
National Healthcare, India |
|
Pharmadocx, Inida |
|
S. Nath & Co., India |
|
Unikal Consultants, India |
|
