|
Why It’s Time to Rethink
How You Assemble Plastic Medical Devices and Components
(Courtesy : Emerson Driven by Innovation, Powered
by Partnership)

Introduction Over the
past several decades, there has been a massive shift
toward the use of plastics in medical devices, respiratory
care systems (oxygen delivery and medical ventilators),
kidney dialysis equipment, surgical instruments, personal
drug delivery devices like insulin pens, inhalers and,
especially recently, in-vitro diagnostic (IVD) testing
equipment for big labs, point-of-care (POC) and at-home
testing. The COVID-19 pandemic created a surge in demand
for personal protective equipment, including masks and
respirators. At the same time,
the market as a whole is exploding. A 2021 report from
Research and Markets predicts the global medical device
market will reach $745 billion by 2030, growing by 5%
annually from 2020 to 2030 owing to aging population
worldwide, the prevalence of infectious diseases as well
as the increasing prevalence of chronic diseases,
technological innovation and penetration of healthcare
insurance. However, as
production volumes increase, quality requirements become
more stringent, and the materials used in devices change,
the traditional methods of assembly like gluing,
mechanical fasteners, and simple welding techniques like
heat-sealing often are no longer adequate.
For Example
Hemodialysis filter housings: Until recently, these
cylindrical housings have been made primarily from
polycarbonate, an excellent, high-performance plastic, and
assembly involved glue and mechanical fasteners. However,
the housings also contain a chemical called bisphenol A (BPA)
that has been linked to adverse health effects.
Consequently, out of an abundance of caution, many
dialyzer manufacturers are shifting to polypropylene, a
polymer that is nontoxic, tasteless, low density,
unaffected by humidity, and relatively inexpensive and
easy to process. Unfortunately, its mechanical
characteristics make it unsuitable for the glues and
screws used with polycarbonate, so manufacturers are
turning to ultrasonic and laser welding instead, with
excellent results.
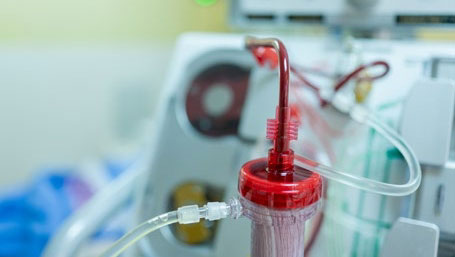
Figure 1. Kidney dialysis machines (dialyzers) incorporate
a filtering cartridge like this one in lower right. They
consist of extremely fine filtration media encased in a
plastic hous¬ing. Image courtesy of Emerson.
Respirator flow sensors:
As the name implies, these components measure the flow of
oxygen to patients receiving respiratory support. These
devices used to be produced in relatively small
quantities, and the two molded plastic halves were
generally glued to¬gether. Gluing was a slow process,
requiring a high level of precision, and rejects were
common, but these limitations were accepted until the
COVID-19 pandemic and other breathing diseases caused
demand for flow sensors — which must be replaced daily —
to rise in the millions. To meet this dramati¬cally
increased demand and control costs, these flow sensors are
increasingly assembled using ultrasonic welding.
Safety syringes:
Single-use syringes have been a ubiquitous element in
healthcare, even more so with the millions of COVID-19
vaccine doses being administered. Many nations mandate the
use of what are called safety syringes, designed to
prevent needlestick injuries and/or reuse. The most common
approach has been to include a sheath or hood that is
clipped to the syringe so that it can be slid over the
needle after use. These clipped-on covers pose a number of
manufacturing challenges and units with a spring that
automatically retracts the needle into the housing after
use have been developed. The spring is retained in the
syringe housing by a small plastic ring that is
ultrasonically welded to the barrel.
In-vitro diagnostics: The market for IVD devices is
growing rapidly in part due to COVID-19 and other
infectious disease but also due to the efficiency of being
able to diagnose cancers, heart diseases, diabetes and
other conditions both in hospital and home settings.
Testing devices comprise two clear mating parts that are
joined together to form fluid paths. In the past, assembly
has been completed us¬ing a costly, time-consuming glue
process, but today high-speed laser, together with
clear-on-clear laser welding technology enables
affordable, repeatable production of high-purity testing
articles in the high volumes required in the coming years.

Figure 2. In-vitro diagnostics (IVD) antigen lateral flow
test Surgical instruments:
This is another rapidly expand¬ing application, where
gluing and screwing have been used historically.
Ultrasonic stud assembly, however, is increasingly
preferred because it is rapid and cost effective, and
creates a hermetic seal that stands up to sterilization.
In addition, by eliminating the need for screws,
ultrasonic welding eliminates the tiny recesses where
bacteria could accumulate.
Market growth and new application trends — togeth¬er with
advancing ultrasonic and laser technology — make it
imperative for device manufacturers to re-evaluate how
their products are assembled.

The Emerson logo is a trademark and service mark of
Emerson Electric Co. Brand logotype are registered
trademarks of one of the Emerson family of companies. All
other marks are the property of their respective owners.
120 Park Ridge Rd., Brookfield, CT 6804, United States
Phone+1 203 796 0355, Fax+1 203 796 2250
www.Emerson.com/Branson |
