|
Chemical Characterization of Polymeric Medical
Devices: Unveiling the Identity and Risks
 |
Dr. Renjith S.
Scientist B, Biomedical Technology Wing,
Sree Chitra Tirunal Institute for Medical Sciences
and Technology, Trivandrum |
Polymeric medical devices
play an inevitable role in day-to-day medical care. We
can observe materials of polymeric origin in a wide
range of medical devices viz. injection syringes,
blood bags, intra-ocular lenses, vascular grafts,
hernia patches, facial implants, catheters, personal
protective equipment (PPE), and many more (Fig. 1).
Every medical device has to undergo strenuous
biological evaluations in line with international
standards to ensure patient safety and avoid undue
health risks. Chemical characterization plays a vital
role in the biological evaluation as well as
toxicological risk assessment of medical devices.
Fig. 1. Representative polymeric
medical devices
Material selection is one
of the first and foremost steps in the development of
medical devices. One should ensure that the material
is fit enough with all the necessary properties and
characteristics encompassing physical, chemical,
mechanical, morphological, electrical, and biological
to be used for the device development. Chemical
characterization enables to ascertain the presence or
absence of necessary properties and also helps to
establish the equivalence of a material with an
established biomaterial. Even though chemical
characterization alone wouldn’t give a complete
evaluation of a medical device; along with
toxicological risk assessment it would enable the
screening of potential materials/devices for clinical
applications and, establish the biological safety of
new or reprocessed medical devices. ISO 10993 series
of standards describes the biological evaluation of
medical devices with direct or indirect contact with
patients. ISO 10993-18 lays a framework for the
chemical characterization of medical devices involving
the identification and qualitative and/or quantitative
estimation of medical device components to identify
biological hazards and to estimate and control the
toxicological risks associated with medical devices.
The flow chart of the chemical characterization
process as per ISO 10993-18 is outlined in Fig. 2.
The first step in the
chemical characterization of a medical device is to
identify the materials of construction. It generally
means the identification of the components with which
the medical device is made (medical device
configuration). The components of a medical device can
be of polymeric, metallic, or ceramic origin. The
detailed configuration of the medical device will give
the chemical identity of the components, the physical
forms in which they exist in the device, and their
geometrical distribution in the device. Further, the
composition of the individual components of the
medical device has to be estimated. It includes the
percentage purity of the component along with the
quantity of impurities, process aids, or additives
present in it. Catalytic residues, sterilization
residues, and possible degradation products are also
to be estimated. For establishing the medical device
configuration and composition, the information can be
gathered (from vendors), generated, and augmented by
using various characterization methods. A complete
list of characterization techniques suitable for
medical device configuration and composition is
available in ISO 10993-18.
When the complete picture
of the medical device (device configuration,
constituent composition, and clinical use) is
available, one should check if there’s an equivalent
device. The biological equivalence of two devices or
materials involves a number of terms such as chemical
equivalence, physical equivalence, material
equivalence, and contact equivalence. If two medical
devices have identical device configurations,
constituent compositions, and processing methods, they
can be called chemically equivalent. Medical devices
with identical configuration, morphology, topography,
and tribology are said to be physically equivalent. If
two devices have chemical equivalence and physical
equivalence, they could be said to have material
equivalence. Similarly, two devices with identical
clinical use and endpoints of biological evaluation,
are said to have contact equivalence. For two devices
to be called biologically equivalent, they should
demonstrate material and contact equivalence. If
the preliminary chemical characterization of the
medical device is successful in establishing its
equivalence with an established device, one can stop
the chemical characterization with the conclusion that
the medical device has an acceptable health risk and
this outcome could be used for biological evaluation
as per ISO 10993-1.
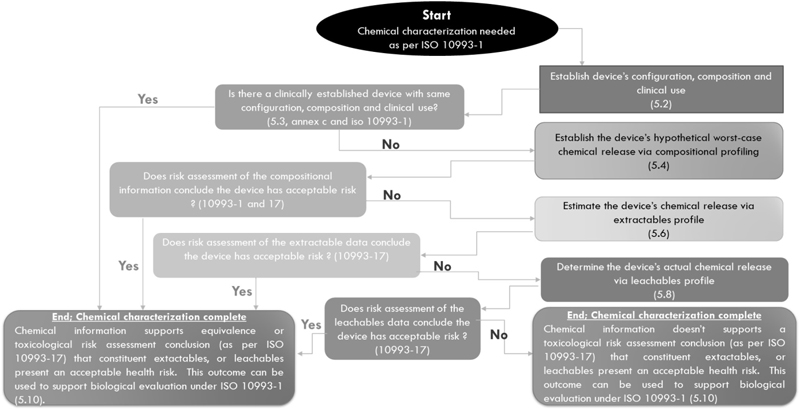
Fig. 2 Flow chart of chemical
characterization process as per ISO 10993-18 |
