|
High Volume Manufacture Of Thinwall
Medical Device Components
With Liquid Crystal Polymer Thermoplastics
(Courtesy : Celanese Chemicals The Chemistry Inside
InnovationTM)
LCP resins are different but not
difficult. Once their polymer characteristics are
understood, they can be put to good use in pushing the
envelope with advanced
designs, part to part tolerance
repeatability, and high volume productivity in the
tens of millions of units … all with the right design,
tooling and processing.
Liquid Crystal Polymer (LCP)
thermoplastics are well-known in the consumer
electronics industry for tight tolerance designs with
high stiffness and strength, plus rapid cycle times
and extreme flow to fill sub-mm wall sections.
Processing benefits allow for micro-molding and
replication of intricate detail in miniaturized
devices for delivery of therapeutic drugs (biological
and pharmaceutical) and vaccines. These same
advantages are translatable to precision combination
drug delivery devices which incorporate complex
mechanisms and wireless connected electronics for
pharma prescription adherence goals.
Specifically, LCP resins can help
designers and engineers achieve more compact,
intricate components through thin-wall molding even as
low as 0.3mm (0.012in) nominal wall without
sacrificing mechanical stiffness and strength as LCP
polymer chains are inherently stiffer & stronger than
many other neat thermoplastics. This can be an
advantage in wearable/on-body devices where
light-weight, compact form factors add value by
creating more free space for pharma container and
other components which can be critical.
Contents
A. Introduction to use of LCP in Drug Delivery Devices
B. Polymer and processing characteristics of Liquid
Crystal Polymers
C. Design benefits from the use of LCP
D. Case study: Vectra® MT® LCP 1305 Lowers Cost Per
Part And Enables Improved Design For Wearable/On-Body
Injector Chassis
A. Introduction to use of LCP in
Drug Delivery Devices
Miniaturization of drug delivery
devices requires careful planning of the integration
of all components into a compact package. This
miniaturization makes wearable devices lighter-weight,
less intrusive and more comfortable for the patient.
In order to provide information on patient usage for
themselves and their healthcare providers, many drug
delivery devices are integrating electronic circuits
and transceivers, in addition to the mechanical
systems to precisely dose the drug. Special materials
capable of molding small and precise components enable
the design of such complex devices with intricate
designs and thinwalls. These benefits significantly
expand the market for controlled dosing products vs.
conventional self administered injections and provide
reliable and precise delivery outside of a clinical
setting. Production of such
devices at a large volume leads manufacturing to
fast-cycling injection molded plastics with high
cavity tools and hot runner systems. Vectra® MT® LCP
provides advantages for each requirement.
• High stiffness, strength and
toughness with long flow-lengths (>150 mm @ 3.2mm) and
capability for wall thicknesses as thin as 0.3 mm at
common device flow lengths and even thinner with short
flow length.
• Ultra-low viscosity at process temperatures for high
flow in thin wall sections.
• Fast crystallization with small mold temperature
excursions between melt injection and part ejection
for fast cycle times and high productivity.
• Tailored dielectric strength for compact integration
of electronic circuits.
• Inherent V0 UL Flammability rating
These attributes make Vectra® LCP the
preferred material for micro connectors and precision
optics for the miniaturization of consumer electronic
devices such as mobile phone cameras and tablet bus
connectors. Combined with Celanese MT® medical quality
management and service package Vectra® MT® LCP brings
these same benefits to the connected and wearable
medical devices.
B. Polymer and processing
characteristics of Liquid Crystal Polymers
LCP resins are unique in the world of
engineering resins. LCP is a high heat thermoplastic,
but its value for most medical devices is its
exceptional flow in thin-wall designs. The morphology
of LCP resin’s nematic rod-like crystalline structure
is very different from amorphous & semi-crystalline
resins.
Even unfilled LCP is extremely strong
& stiff, behaving like a selfreinforcing polymer,
similar to or exceeding mechanicals of 20- 30% glass
fiber composites.
The nematic structure of LCP helps
with its extremely high flow in thin-wall geometries.
High flow combined with high stiffness & strength
allows designers to make thinner structures without
sacrificing structural performance, enabling smaller
parts & freeing up more internal space for pharma or
other components. LCP flows better under high shear
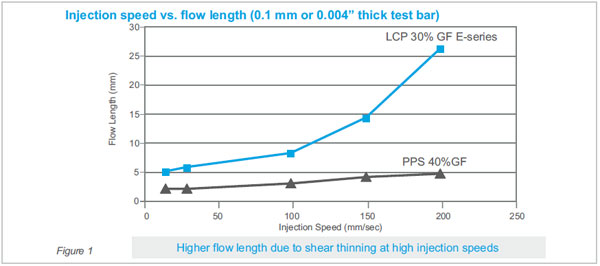
without degrading mechanical properties. Figure 1
shows how LCP flow increases exponentially with high
shear (high injection rate) compared to PPS.
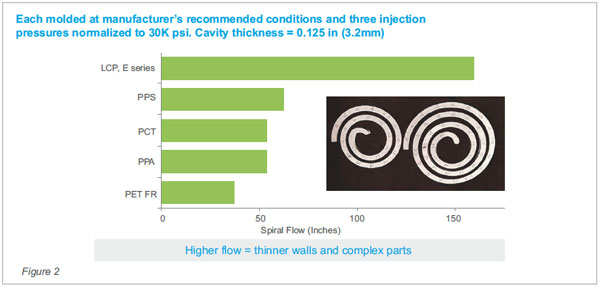
Figure 2 compares spiral flow of 30%GF
LCP vs other 30-40% GF resins at 3.2mm (0.125”).
Each molded at manufacturer’s
recommended conditions and three injection pressures
normalized to 30K psi. Cavity thickness = 0.125 in
(3.2mm)
As seen in Figure 3, LCP has a low
heat of fusion from its ordered molecular structure,
which changes very little from molten to solid phase,
it rapidly solidifies when flow ceases allowing a
rapid cycle from melt injection to part ejection.
Part and tool design must provide for
high enough shear to maintain a low viscosity
throughout the tool. Areas where the flow speed drops,
such as sharp changes in runner direction, can allow
cold slugs to form.
Rapid cycling means more parts per
hour, so fewer molds may be required for high volume
production. Rapid solidification allows for minimal
part flashing. LCP exhibits a melting range rather
than a sharp melt point. The typical solidification
time is a few seconds, with injection molding cycle
time ranges commonly 5 to 15 seconds for small part
molding, depending on the number of cavities.
Since high mechanical shear is used to
thin the resin to make it flow better, high mold
temperatures are not necessary as with polyphenylene
sulfide (PPS) or polyetheretherketone (PEEK). LCP can
be processed at mold temperatures less than 100 °C
similar to many engineering resins and requiring only
water based cooling. Also, contrary to intuition,
smaller gates and thinner runners are better to
increase shear rate and thus flow.
Other characteristics of LCP
include:
• Excellent dimensional stability with
low moisture absorption (0.03%) & low mold shrinkage
(0.1- 0.4%)
• Inherently flame retardant without FR additives
• Very clean, low extractables & ionics, food contact
compliant, biocompatible with MT® grades being FDA
master file listed
• Service temperature from -190 °C up to 240°C, short
term up to 340°C
• High tensile strength (to 185 MPa) & high tensile
modulus (to 30000 Mpa)
• Excellent barrier property to both oxygen and
moisture, one of the best resins
• Sterilizable by steam, ethylene oxide, gamma
radiation or H2O2 plasma
• Excellent chemical resistance
• Natural color is opaque, off-white ivory with
options for coloration.
To support customer applications in
regulated medical devices, Celanese provides
regulatory and quality management support to enable
customer compliance to ISO 13485. This includes
elements related to Celanese polymers, ingredients,
manufacturing controls and supply chain management.
Celanese MT® polymers service
package
• Material compliance to FDA and EU
requirements
• Long-term supply assurance without change of
formulation
• Animal- and latex-free formulations
• Certified biocompatibility (USP 23 Class VI / ISO
10993, etc)
• FDA Drug & Device Master Files |
