|
NMDPC : To Catalyse
Growth of Medical Device Sector in India
 |
Dr. Jitendar Sharma
Member Secretary, National Medical Devices Promotion
Council and MD & CEO AMTZ |
An Introduction to NMDPC: The
National Medical Devices Promotion Council (NMDPC) has
been set up as a catalyst-organization for facilitating
and promoting the Indian Medical Device Industry, under
the aegis of Department for Promotion of Industry and
Internal Trade (DPIIT) on 7th December 2018, with Andhra
Pradesh MedTech Zone (AMTZ) as its
technical secretariat. On 14th of December 2018, the then
Hon’ble Minister of Commerce & Industry inaugurates NMDPC
at the “4th WHO Global Forum on Medical Devices”, held at
the AMTZ Campus, Visakhapatnam, Andhra Pradesh.
NMDPC with its core objective to
promote and facilitate the Medical Device Industry and
create a nurturing ecosystem (which the sector lacked), is
poised to be the National Forum now to discuss and consult
stake holders of the Medical Device ecosystem (ranging
from policy makers, start-ups, incubators, R&D
institutions-to-manufacturers of vivid size and
capabilities) and partner with organizations of
international repute, who can add value and prescribe
solutions pertaining to growth of medical
device manufacturing and exports.
As the apex Council for facilitating
and promoting the Medical Device industry and to position
India as a pioneer in Medical Device and Health technology
space, following are some key activities undertaken by the
council:
• Policy Facilitation
• Strategic Forums (Policy, Best Practices, Partnerships)
• Dissemination of International Norms
• Industry Support (Manufacturing, Regulatory Challenges,
etc.)
• Market Access
Boosting Indigenous
Manufacturing-Enabling Level Playing Opportunities to
Harness True Potential: Since inception, the council
has been interacting closely with the stake holders from
government, as well as private sectors (manufacturer/
supplier), healthcare providers and patient groups. It was
found that initiatives that are directed towards
benefitting the medical device manufacturers with respect
to Preference to Make in India, Order 2017 or through the
implementation provisions from Department of Pharma were
in a way not followed in heart and spirit and doesn’t
reflect in the tenders floated by many State & Central
Government Agencies. Additionally, the Make-in-India
proposition may also take a back seat if timely payments
are not released to the manufacturers or suppliers whose
payments are pending for year/s together from the State/
Central Health Corporations. Though the manufacturers are
integral part of the value chain, however such
discouraging instances may lead to a severe cash crunch to
them or land them in bankruptcy or put a threat to their
existence or sustainability of their existing projects,
performing R&D, finding markets, commissioning newer
initiatives, all of them can take hit for the same reason.
Tender Deviations:
NMDPC has reviewed more than 200 odd tenders across the
country from the period April 2019 to July 2019, and have
found 38% of them deviating in terms of mandating foreign
regulations or some of them are even upright asking
specific company for supplying or mentioning their brand
name, thus killing fair competition and are once again not
adhering with General Financial Rules, 2017 (GFR)
guidelines. Such tenders are possibly biased, and are
against the larger interest of the manufacturing
community, especially narrowing the scope of Indian
manufacturers to participate in the state/ central led
procurement process.
Table1: Total number of
Tenders studied from April to July 2019
| No. |
Period |
Deviations |
Without Deviations |
Total Tenders |
| 1 |
1 - 30 April 2019 |
8 |
15 |
23 |
| 2 |
1 - 15 May 2019 |
8 |
16 |
24 |
| 3 |
16 - 31 May 2019 |
16 |
41 |
57 |
| 4 |
1 - 15 June 2019 |
11 |
13 |
24 |
| 5 |
16 - 30 June 2019 |
11 |
20 |
31 |
| 6 |
1 - 15 July 2019 |
8 |
15 |
23 |
| 7 |
16 – 31 July 2019 |
18 |
12 |
30 |
| |
Total |
80 |
132 |
212 |
| |
Total Percentage of
Deviations |
38% |
| |
Total Percentage without
Deviations |
62% |
From Table 1, the
percentage of tender deviation is approximately 38%
accounting to 80 tenders which are asking exclusively for
foreign regulatory approvals in India. Of these deviations
the GoI or Central government organizations have shown
overall 26% deviation while Haryana state government alone
has shown about 20% deviation as shown in graph 1 below.
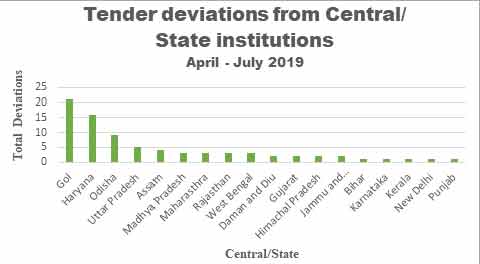
Graph 1: Tender
Deviations amongst State and Central organizations
Considering the high
value and importance of medical device equipments in the
field of Radiology and Cardiology, NMDPC has further
assessed deviation of tenders into three categories,
namely – 1. Radiology, 2. Cardiology, and 3. Others. It is
found that of the total deviations 36% of tender
deviations fall under Radiology and Cardiology categories.
Way Forward:
Transparent procurement practices with adherence to Public
Procurement Order 2017 can open a lot of avenues for
Indian manufacturers, provided payments are also settled
with the manufactures in a timebound manner. Secondly,
National Medical Devices Promotion Council realizes that
there is a distinct ecosystem advantage which can be
provided to the medical device stakeholders while
operating out of a cluster, considering Andhra Pradesh
MedTech Zone (AMTZ) as a model cluster where common
resources like infrastructure, scientific facility,
testing facility, sterilization facility, regulatory
enablement and market access are in complete synchrony
with manufacturing. Replicating such clusters and linking
them with each other or linking them to the existing
clusters for enabling smooth flow of services can go a
long way as far as import substitution is concerned, and
thus making the best use of the capabilities which already
exists..
|
