|
Medical Device Sterilisation : Key Essentials, Options And
Challenges
|

Mr. Amit Shrivastava
- Facility-Incharge
ISOMED, Board Of Radiation And Isotope Technology
Department Of Atomic Energy, Government Of India
BARC, TROMBAY, MUMBAI - 85. |
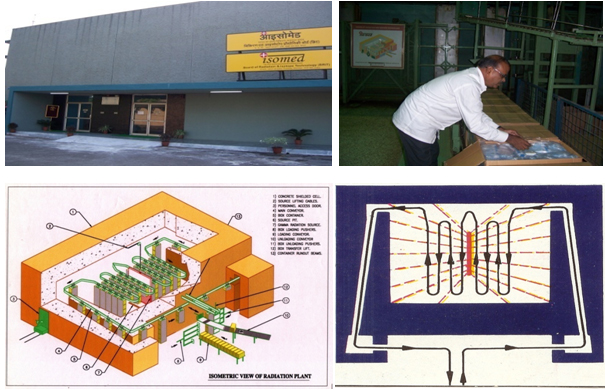
Gamma Radiation
Sterilisation: The Indomitable Workhorse For Terminal
Sterilisation Of Medical Products
-
This article deals with
the packaging requirements for the radiation
sterilisation, the product range, limitations of the
technology and the future prospects..
-
In India, market share of
ETO and Gamma sterilisation is 80 and 15 percentage
approximately whereas sterilisation by Autoclaving is
limited to 5 percentages.
The
cornerstone for demonstration of techno economic
viability of the technology of Gamma Radiation
Sterilisation in the country was laid nearly four
decades ago on January 1, 1974 by commissioning of the
ISOMED (Irradiation Sterilisation of Medical Products)
facility by the Department of Atomic Energy,
Government of India. During the course of over four
decades of marathon service to the healthcare
industry, the facility has established a niche of it
and is popularly recognized as a centre of excellence
in the field of radiation sterilisation in the Asia
Pacific region. Since its inception, the facility
hitherto has processed an astronomical volume
admeasuring 0.2 Million Cubic Meters approx of medical
products.
The reservoir of technical
expertise cherished by the facility that supplemented
by the exemplary legacy of customer oriented services
to the facility users has ushered into emergence of
similar myriad facilities in the private sector from
the year 2005 onwards. The growing level of awareness
about the radiation sterilisation in the health care
industry justifies without an iota of doubt, the
mission behind setting up of the ISOMED. |
As far as relative
apportionment of various industrial methods of
sterilisation is concerned, market share of ETO, Gamma and
Electron Beam on international scale is approximately 60,
30 and 2 percentage respectively. However in India, market
share of ETO and Gamma sterilisation is 80 and 15
percentage approximately whereas sterilisation by
Autoclaving is limited to 5 percentages.
Recent studies in the country
have indicated that sterilisation techniques such as ETO
and autoclaving bear the higher risks with respect to
morbidity and mortality in the hospitals due to infection.
It is a pressing need to resort to only gamma radiation as
far as sterilisation of the gauge, cotton and linen is
concerned particularly under Indian circumstances.
Accordingly Board of Radiation and Isotope Technology, a
constituent industrial unit of Department of Atomic Energy
has taken up an initiative, to address the burgeoning
issue of morbidity and mortality in the hospitals in
India. The work regarding conceptual design of compact
gamma irradiators based on Co-60 radioisotope for Central
Sterile Supply Departments (CSSDs) of hospitals (100 beds
capacity or more) had been taken up early 2012 and
currently a prototype 1:2 scaled down model (fully
functional) is available with BRIT.
The following paragraphs while
highlighting the decade wise performance of the ISOMED
facility would attempt to reflect upon the genesis of the
technology of radiation sterilisation in the country from
a TLC (Technology Life Cycle) perspective.
Decade I (1974-1984): The
Era Of Technology Introduction
The core focus during the
decade was on sensitizing the health care industry about
this relatively novel technology. The prime stake holders
of the facility were consulted on several aspects of the
radiation sterilisation including the technology knowhow,
material compatibility issues, and the validation of
radiation sterilisation process. For dissemination of the
technology, many technical workshops, with the
collaboration of IAEA (International Atomic Energy Agency)
were successfully conducted. The CSSDs (Centralized
Sterile Services Departments) of the various hospitals
across Mumbai were offered novel door to door
sterilisation services. The in-house production of the
radiation sterilised maternity kits was also undertaken.
The radiation dosimetry mainly resorted to the Perspex
dosimeters using the spectrophotometric measurement
method. The statutory licenses from the Maharashtra state
drug control department were obtained for the radiation
sterilisation of the products as described in the Drug and
Cosmetics Act 1940 and rules there under. The facility had
been successful to a larger extent in sensitizing the
health care industry till end of this decade.
Decade II (1984-1994): The
Era Of Technology Ascent
The decade had been bubbling
with the activities primarily concerned with the
development of radiation stable polymeric configurations
in technical collaboration with BARC , to be used as
Single Use Disposable Medical Devices in the state of the
art Research and Development labs (Polymer Testing/
Microbiology Lab). It was during this decade major
healthcare establishments in the country viz. M/S Johnson
and Johnson Ltd., M/S Glaxco Smithkline, M/S Hindustan
Unilever Limited , M/S
Hindustan Latex Limited ( now known as HLL Life Care
Limited ) etc. included ISOMED facility in their critical
supply chain as a contract gamma radiation service
provider for terminal sterilisation of their products. The
technology was being well accepted by the industry and the
facility depicted encouraging performance on the
commercial front.
A paradigm shift in the dose
measurement system occurred in form of the chemical
dosimetry (Cerric Cerrous – Potentiometric Dose
Measurement System). By the end of this decade, the
facility had acquired a reasonable clientele.
Decade III (1994-2004): The
Era Of Technology Ascent (Contd.)
The production and supply of
the chemical dosimeters received an impetus. The radiation
sterilisation process validation was standardised and the
facility had gained a substantial expertise in this
crucial aspect of radiation sterilisation technology. The
successful operation of newly commissioned facilities by
other governmental / non governmental agencies heralded
the news of technology making its vital presence amongst
the stakeholders from the healthcare industry. R&D trials
for the indigenous
development of the qualitative indicators of radiation
processing were also undertaken. The facility had been
availing itself of the International Dose Assurance
Services (IDAS) – IAEA for the quality accreditation of
its indigenously produced chemical dosimeters. By end of
this decade the facility had depicted a spiral growth both
in terms of quantum of the product processed as well as
the revenue earned. The technology had also entered into
the early phase of its maturity.
Decade IV (2004-2014): The
Era With Early Phase Of The Technology Maturity
The era began with a sudden
spurt in number of radiation processing facilities in the
private sector thus widening the market share of this
technology for the healthcare sector. ISOMED shifted its
core focus to benchmarking its quality with respect to
radiation sterilisation services and adding innovative
safety features. The innovative 24x7 automatic consignment
processing status enquiry system along with Radio
Frequency enabled Access Control features for the
irradiation cell won accolades from the stakeholders of
the facility. Facility received eu GMP compliance status
from the MHRA (Medicine and Healthcare Regulatory
Authority) - UK and was recognized as an accredited
foreign device manufacturer from the govt. of Japan.
Facility also acquired ISO 9001:2008, ISO 13485:2003, ISO
11137:2006, and OHSAS 18001 : 2007 accreditations thus
railroading its perpetual unwavering commitment of
benchmark radiation sterilisation services to the
healthcare industry.
Packaging For Gamma
Radiation Sterilisation
The success of the radiation
sterilisation has a pivotal bearing on the packaging
integrity of the products. It is the regular industry
practice that the products are packed firstly using the
primary packaging and then a secondary packaging is
provided in form of fibreboard cartons for facilitating
the smooth shipment of the products from the primary
manufacturer’s site to the contract radiation service
providers. The primary packaging should provide a complete
barrier to the entry of microorganisms and
should be designed to facilitate aseptic removal of
contents. The materials to be sterilised are packed in
impermeable films for example, polyethylene,
cellophane-polyethylene or paper-polyethylene laminates,
which can be heat sealed thus ensuring maintenance of
sterility. These laminates have good tear and impact
strength, have customer appeal and are inexpensive. Other
types of laminates can be designed for convenience and to
suit the product. Unsupported polyethylene films of 300
gauge thickness are suitable for soft products and of 500
gauge for rigid products. The exhaustive information on
the packaging aspects for the products to be radiation
sterilised are available in the website of M/S Board of
Radiation and Isotope Technology, Department of Atomic
Energy, Government of India at
www.britatom.gov.in.
Page
1 :
2 :
3 :
4 :
5 :
6 :
7 |
