|
Development of Plastics Injection Molded Medical Device: A
Systematic Approach is Key to Success
Brainstorming sessions are
often held during this stage of development with members
of Design team, R&D, marketing, and physician consultants.
Computational analyses, such as stress and flow studies,
are conducted to further understand the behavior of a
proposed device. The team often develops a 3D CAD model of
a proposed device, which subsequently forms the basis for
the construction of physical prototypes. Design reviews
(per 21 CFR 820.30) with all cross-functional team members
done to systematically assess a deviceís design progress.
Risk management is a critical
component of the analysis, prototype, and design
development phase. The FDA expects companies to have a
complete risk-management plan in place, which consists of
the two aspects of risk analysis (identification and
quantification of risks) and risk management (mitigation
of the identified risks).
Just because part looks good
on paper, doesnít mean that it will turn out well in
production. Beyond merely creating an aesthetic and
functional design, experienced engineers understand the
importance of optimizing a part for manufacturability. DFM
is very crucial to be considered while designing any new
medical device. Itís essential to Design the product For
Manufacturability as, about 70% of manufacturing cost of a
product (Cost of materials, processing, Assembly) are
determined by design decisions.

Design Verification, Design
Validation, Process & Tooling Development :
Design Verification is
critical to ensure that output of a design stages meet
design input requirements and itís mandatory for
regulatory approvals. DFMEA is also done to ensure
anticipated design deficiencies have been detected and
corrected (before release of design) by the end of this
process.
After the team finalize the
device concept and tests several prototypes, final
engineering drawings are created. Designer generates
formal manufacturing drawings for the new device,
consisting of component and assembly-level drawings. Final
prints must conform to geometric dimension and tolerance
standards to ensure that design requirements are
effectively communicated to suppliers and manufacturers.
Tolerance stack-ups analysis
are also conducted on the final design to ensure that
there are no mating-part interferences in a device, or
between a device and another instrument with which the
device interacts. Material specifications, packaging
drawings, and marking and labeling specifications are also
finalized.
Proto Tooling For Device
Validation :
Proto tool which is a replica
of the production tool on smaller scale is been made to
give realistic enough to try out ideas before making a
significant investment and thus help preventing costly
changes to the hard tool once the product is produced.
Itís recommended to use
scientific injection molding process to evaluate the
molding process and make any necessary adjustments. This
provides a consistent, repeatable production of the part.
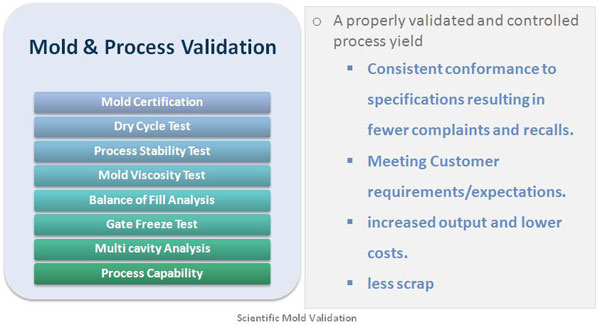
Through this process, process engineers determine both the
optimal molding conditions and the molding window, or the
best speed at which plastic should be injected. Using
real-time production monitoring systems and advanced
quality inspection equipment, the process also examines
how easily the part can be manufactured and how
consistently the mold runs, based on several criteria.
Parts produced from validated proto tools have to be
verified and validated as per the verification and
validation plan.
Design Validation which is process of checking and
establishing whether device design specification meets the
user need and comply statutory requirements for intended
use is been done as per the validation plan. Device
produced from proto tool can be used for clinical trials
and regulatory approvals.
Page
1 :
2 :
3 :
4 :
5 |
