| Category of gamma
Irradiator • Category I: self
contained dry storage
• Category II: panoramic dry storage
• Category III: self contained wet storage
• Category IV: panoramic wet storage
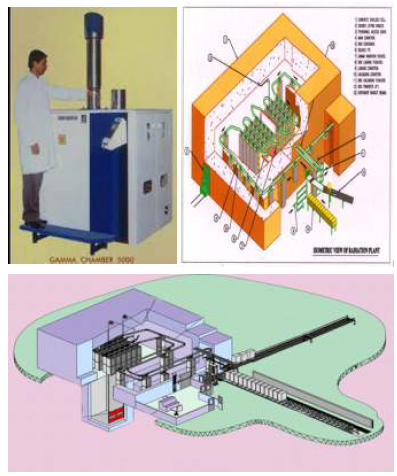
Gamma Irradiator Sources
• Co -60 (average
photon energy: 1.25 MeV & half-life: 5.27 y used
in almost all industrial radiation processing
facilities)
• Cs –137 (photon
energy: 661.7 keV & half-life: 30.1 y used in
small, self-contained dry storage irradiators, for
irradiation of blood and insect sterilization)
ISOMED – India's
First gamma Radiation sterilization facility
• ISOMED the first
radiation plant for sterilization of medical
products, was set up in India in the year 1974 by
Department of Atomic Energy at Trombay Mumbai with
assistance of UNDP. Setting up of ISOMED had
ushered in the radiation sterilization era in the
country.
• In the last four
decades of operation, ISOMED served over 1600
manufacturer of medical products and
pharmaceuticals in the country.
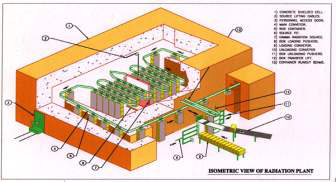
ISOMETRIC VIEV OF
ISOMED Plant
PRODUCT FLOW IN
IRRADIATION CELLISOMED
 |
ISOMED FACILITY IN
OPERATION

ISOMED plant
• The Plant Essentially
consists of
• Radiation source Co-60
• An automatic system for conveying the product
boxes into the irradiation cell exposing to the
radiation field for a specified period and taking
them out of the cell
• Laboratory for dosimetry to release the
processed product
• Facility for production of Radiation Indicator
button
Procedure for radiation
sterilization of licensed products
• Customer must have a valid
manufacturing license issued by FDA/CDSCO for the
products
• A Quality Agreement between ISOMED and Customer
• Customer must obtain a loan license in favor of
IOSMED for the products intended to be sterilized
by gamma radiation
• After scrutiny of application, ISOMED would
issue a consent letter in prescribed form to the
applicant to submit to CDSCO/state FDA for getting
loan license.
Procedure for radiation
sterilization of non licensed products
• Customer must have a valid
manufacturing license issued by competent
authority for the products
• A Quality Agreement between ISOMED and
Manufacturer
Requirements for
Sterilization by gamma radiation
AERB approved radiation
facility
• Radiation Source (Co-60) to
deliver desired radiation dose
• AERB approved staff
• Primary Packaging : The packaging should provide
a complete barrier to the entry of microorganisms
and should be designed to facilitate aseptic
removal of contents. Unsupported PE films of 300
gauge thickness are suitable for soft products and
500 gauge for rigid products.
Radiation dose (kGy)
• The energy of radiation is
deposited in medium when radiation passes through
medium. Radiation energy absorbed is called
radiation dose and is measured in Grey or kGrey
• Survival of micro-organism by radiation depend
on D10 value of micro-organism, environmental
conditions (humidity, temperature, water)
• Dose measurement - Chemical method:
• Ceric- cereous dosimetry system |
